mainmenu
Research Highlight
The Capture and Release of Nitric Oxide Using N-Heterocyclic Carbene
6. The Capture and Release of Nitric Oxide Using N-Heterocyclic Carbene
Nitric oxide is one of the major environmental pollutants. Being highly reactive to oxygen, nitric oxide destroys the ozone layer or is converted to nitrogen dioxide, which in turn oxidizes to nitrate ions, causing acid rain. Since 1979, however, nitric oxide has drawn renewed attention as its various physiological effects were revealed. We recently succeeded in saving and transporting nitric oxide by chemically fixing it using an organic compound called N-heterocyclic carbene.
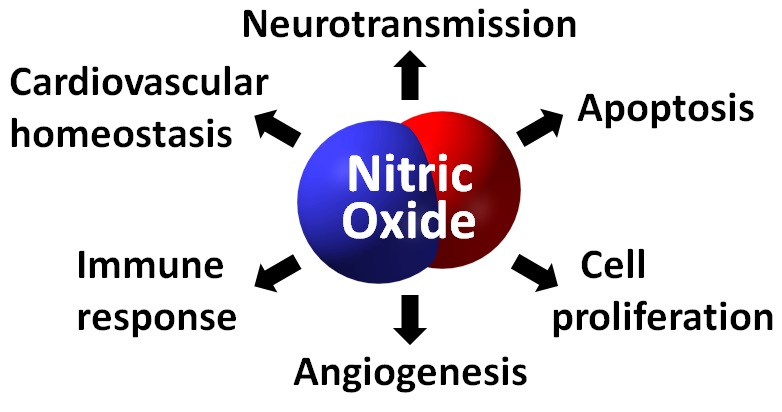
Nitric oxide is involved in various body reactions such as vasodilatation, relaxation of muscle cells, angiogenesis, immune responses, and neurotransmission. As these effects were discovered, nitric oxide started to be used in the medical field. Nitric oxide is readily converted to nitrite or nitrate in a living body due to its high reactivity. In order to have substantial clinical effects, nitric oxide should be sent where it is needed without any loss. For this reason, recent research has focused on carriers that can capture and release nitric oxide on demand. To address the aforementioned issues, we used an organic compound called N-heterocyclic carbene (NHC). NHC is well-known to stabilize unstable molecules like radicals. NHC is a collective name for unstable intermediate compounds containing divalent carbon atom with nitrogen heterocycle. While reactivity between NHC and nitric oxide had not previously been reported, we explored the unknown reactivity and succeeded in obtaining N-heterocyclic carbene nitric oxide (NHCNO), a solid organic matter, by adding nitric oxide gas to NHC. X-ray diffraction analysis of NHCNO showed that nitric oxide was directly bonded to the carbene center in NHC. Electron paramagnetic resonance spectroscopy showed that NHCNO had not lost the radical character. This means that the nitric oxide bonded to NHC maintains its unique chemical properties. Nitric oxide reacts to oxygen in the air and changes to nitrogen oxide within a few seconds. However, NHCNO synthesized by our team was verified to be stable through various experiments, even under air and moisture at room temperature. This means nitric oxide can maintain its reactivity for a relatively long time, and thus NHC is an ideal carrier of nitric oxide.
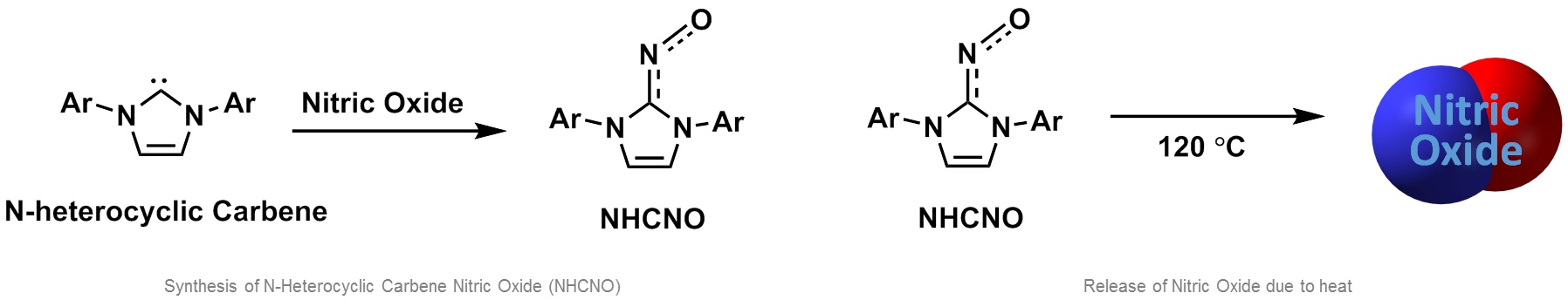
A carrier should be able to absorb or release matter when necessary. We verified this feature through experiments and the reaction mechanism studies using density functional theory. It was found that the bonded nitric oxide was released when NHCNO was heated, meaning the NHCNO compound has high availability as a candidate material for new medicine delivering nitric oxide to the desired place at the desired time. This research is a significant example of the carbon of N-heterocyclic carbene acting in a similar way to transition metals. This similarity would be better understood through future research on the compound’s reactivity with various small molecules. We are planning to conduct further research to find ways to utilize the compound for new medicine development and medical purposes.
Park, J.; Song, H.; Kim, Y.; Eun, B.; Kim, Y. Bae, D. Y.; Park, S.; Rhee, Y. M.; Kim, W. J.; Kim, K.; Lee, E. “N-Heterocyclic Carbene Nitric Oxide Radicals” J. Am. Chem. Soc. 2015, 137, 4642-4645.