mainmenu
Research Highlight
The Development of Highly Stable, Water-dispersible Metal Nanostructure Using Cucurbituril Nanocapsules
3. The Development of Highly Stable, Water-dispersible Metal Nanostructure Using Cucurbituril Nanocapsules
Catalysis is a core technology in the field of chemical industry. This is because catalysts are essential for efficiently promoting chemical reactions. In practice, 85% of chemical processes use catalysts. However, catalysts can lead to safety and economic problems, as well as causing environmental damages. This is because catalyzed reactions use highly toxic solvents such as toluene or hexane, and reaction conditions, including humidity and temperature, are strictly controlled. Over the past decades, studies have been conducted from various perspectives to develop catalytic processes without using toxic solvents in order to improve the aforementioned problems. In recent years, our center has been receiving a lot of attention after the announcement of breakthrough results that can contribute to the development of eco-friendly catalysts.
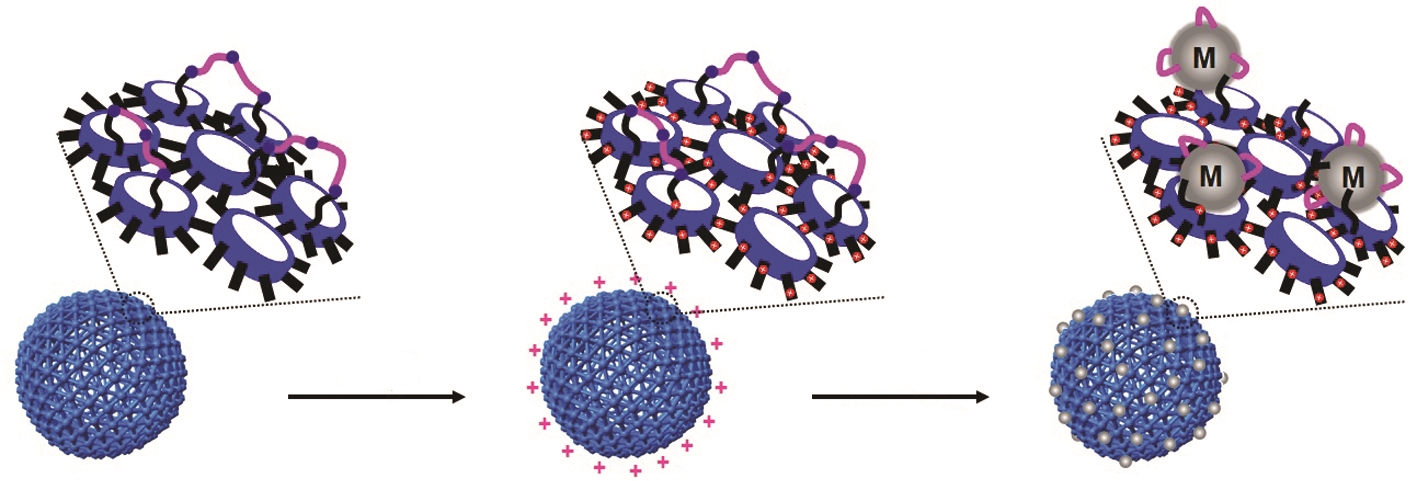
Metal nanoparticles have wider surface areas compared to their volumes and have advantageous features such as excellent quantum confinement effects in relation to their size and surface plasmon effects. For these reasons, they are often used as catalysts in pharmaceutical industries and fine chemistry. However, toxic solvents are essentially required in the catalytic processes, making them costly and harmful to the environment due to their low stability, low degree of dispersion, and continuous leaching. As an alternative, a process that uses eco-friendly solvents such as water instead of organic solvents can be considered. Cucurbit[6]uril can selectively bind to further developed on this and predicted that metal nanoparticles can be uniformly grown on the nanocapsule. To prove the hypothesis, we synthesized water-dispersible uniform-size nanocapsule with positively charged the surface which allowed selective gathering of metal ions with negative charge on the surface of nanocapsule by injecting metal salts. The results of these processes confirmed a unique structure wherein metal nanoparticles grew only on the nanocapsule surface, and showed that the structure was stabilized by the cucurbit[6]uril and disulfide loops. Furthermore, the synthesized metal nanostructure was stable in water for more than six months. The chemical species and can ensure the stabilization of metal nanoparticles without aggregation. In 2007, we synthesized hollow spherical nanocapsules using cucurbit[6]uril. The surface of the synthesized nanocapsules consisted of cucurbit[6]uril units and disulfide loops in a trap-shape exposed on the surface, and these can strongly bind with metal nanoparticles. We size and type of the metal nanoparticles were successfully adjusted according to the concentration and the type of metal salts used. C–C and C–N bond formations are the most commonly used reactions in pharmaceutical and fine chemical processes. We performed these reactions in aqueous solution using metal nanostructures. The results confirmed that the metal nanostructures have excellent stability and high catalytic activity, and can be recycled many times. Interest in eco-friendly processes has increased recently as the environmental damages have emerged as an important issue worldwide. Hence, this study can provide a new beginning and inspiration to green chemistry.
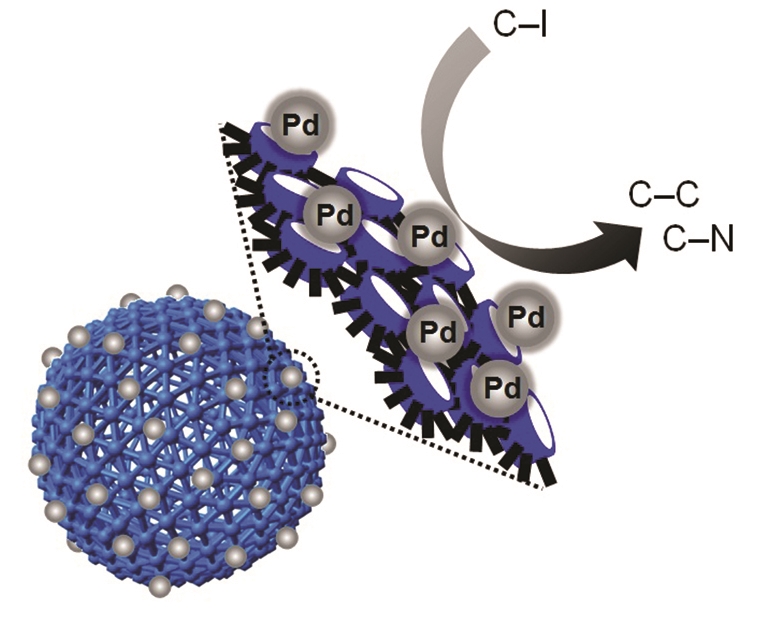
Yun, G.; Hassan, Z.; Lee, J.; Kim, J. Lee, N.-S.; Kim, N. H.; Baek, K.; Hwang, I.; Park, C.; G.; Kim, K. “Highly Stable, Water-Dispersible Metal-Nanoparticle-Decorated Polymer Nanocapsules and Their Catalytic Applications” Angew. Chem. Int. Ed. 2014, 53, 6414-6418.