mainmenu
Research Highlight
High Affinity Host–Guest FRET Pair for Single-Vesicle Content Mixing Assay: Observation of Flickering Fusion Events
2. High Affinity Host–Guest FRET Pair for Single-Vesicle Content Mixing Assay: Observation of Flickering Fusion Events
Single vesicle fusion assays using SNARE-reconstituted vesicles have played a vital role in elucidating the mechanisms of SNARE mediated vesicle fusion by allowing controlled observations which are not possible by in vivo studies. However, the existing assays have limitations such as low sensitivity and limited ability to detect transient fusion pore openings. Our group in collaboration with Prof. N. K. Lee group in POSTECH have developed a novel in vitro assay that allows them to study the dynamics of vesicle fusion. With the assay, the researchers show that the vesicle fusion process, guided here by neuronal SNAREs, undergoes “flickering” for the first time.
Many biological processes rely on tiny cargocarrying sacs called vesicles. Neurotransmitter release, exocytosis, vesicle trafficking, and other biological processes depend on the fusion of these phospholipid vesicles to each other or to a larger cell. The fusion is facilitated by proteins called SNAREs(soluble N-ethylmaleimide-sensitive factor attachment protein receptor) that work like tethers. Although researchers have spent years investigating how SNAREs and other regulatory proteins govern vesicle fusion, many details are unknown. Single vesicle fusion assays using SNARE-reconstituted vesicles have played a vital role in elucidating the mechanisms of SNARE mediated vesicle fusion by allowing controlled observations which are not possible by in vivo studies.
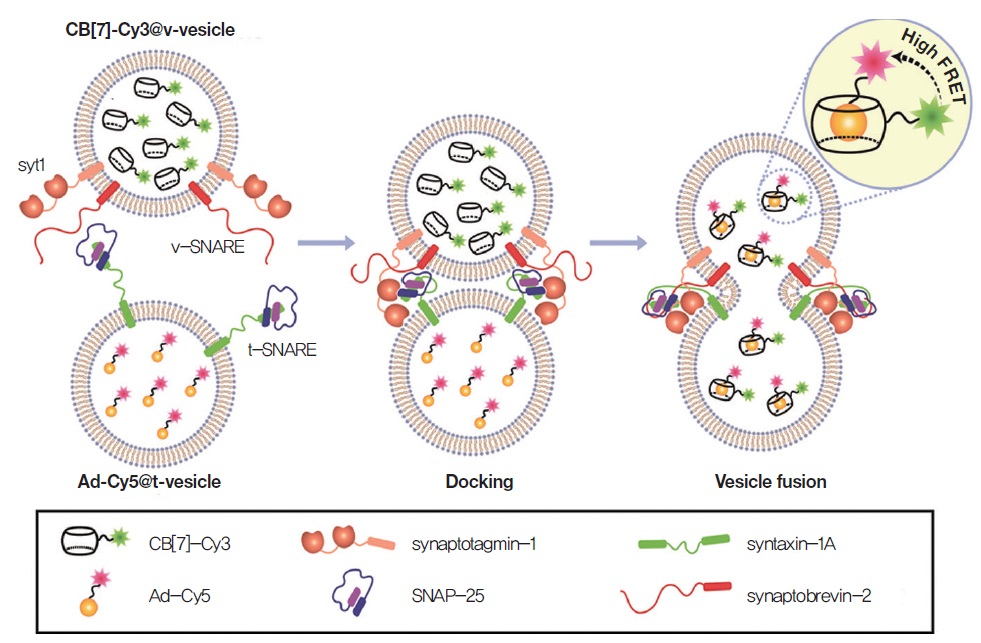
However, the existing assays have limitations such as low sensitivity and limited ability to detect transient fusion pore openings. To overcome such limitations, our group in collaboration with Nam Ki Lee group has recently developed an in vitro assay that allows them to study the dynamics of vesicle fusion. We synthesized a host-guest fluorescence resonance energy transfer (FRET) pair (CB[7]-Cy3 & Ad-Cy5) displaying unique features such as small size, high affinity and high signal-to-noise ratio. With the assay, we discovered that our assay has the ability to distinguish between the docking and membrane fusion pore opening state with a high signal to noise ratio. Remarkably, CB[7]-Cy3 & Ad-Cy5 FRET sensor with a high signal to noise ratio allowed us to observe, for the first time, reversible fusion pore flickering events, i.e., a repetitive opening and closure of the fusion pore in an in vitro content-mixing assay. This work demonstrated the potential of our novel content-mixing assay for the development of synaptic vesicle fusion study. This synthetic “host-guest FRET pair” with extremely strong binding affinity and specificity can be extended to the analysis of other short-lived and dynamically rapid biological processes and may be applied to various chemical and biological studies as a supramolecular beacon.
Gong, B.; Choi, B.-K.; Kim, J.-Y.; Shetty, D.; Ko, Y. H.; Selvapalam, N.; Lee, N. K.; Kim, K. “ High Affinity Host–Guest FRET Pair for Single-Vesicle Content-Mixing Assay: Observation of Flickering Fusion Events” J. Am. Chem. Soc. 2015, 137, 8908-8911.